The Stakes of Stimulants: How Amphetamine Misuse can Induce Psychosis in a Collegiate Environment
Michael Silva
Illustrations by Michael Silva
You’re a college student cramming for finals. Your coffee is starting to wear off and sleep is nowhere in sight. A friend hands you a small pill, saying it’ll help you focus and stay alert, so you take it. It sounds like a quick fix, harmless even. Or suppose you’re the friend; you’ve been prescribed this medication and you figure starting to double up when you need an extra boost couldn’t hurt. What you may not realize is that such a seemingly inconsequential choice could actually lead to paranoia, confusion, or even psychosis [1, 2]. Semesters or even lives could be lost. In high-stakes college environments, stimulant misuse is more common than one might think, and the effects can be devastating [3, 4]. We hope to unearth the hard truths of stimulant misuse and the heightened vulnerability of college students to its effects by examining its neurochemical mechanisms, symptoms, and risk factors, as well as its overlap with primary psychotic disorders.
Deconstructing the Delusions: What are Psychoses and Psychotic Disorders?
Psychosis is a general term for a collection of distressing symptoms that can contribute to various psychiatric conditions [5, 6]. Despite not being classified as a disorder itself, psychosis is a common component of psychotic disorders such as schizophrenia [5, 6]. Psychotic disorders are characterized by disturbances in five main categories: delusions, hallucinations, disorganized thought, disorganized behavior, and negative symptoms [7, 8]. A person experiencing a psychotic disorder might develop thought patterns that are repetitive or nonsensical and may move quickly from topic to topic due to disordered thinking [5, 8]. Disordered thinking can also be accompanied by disorganized behavior, like unpredictable mood swings or decreased reactivity to one’s environment. Psychotic disorders are also marked by delusions — fixed, false beliefs that persist even when met with contradictory evidence. Examples of delusions include: the persistent belief that someone is being watched through their mirror, or the conviction that the people on television are speaking directly to them, conveying cryptic messages [5, 8]. Similarly, a person experiencing a psychotic disorder may have hallucinations, which are falsely manifested and perceived sensations such as hearing music playing in a silent room or feeling someone’s breath on the back of their neck when no one is there [6, 8]. Disorganized thinking and behavior, delusions, and hallucinations are categorized as positive symptoms since they all ‘add’ something to a person’s perceived experience [5]. Psychotic disorders also include symptoms that ‘subtract’ from normal behavior or experience — such as dampened emotional expression, a lack of interest in socializing, or a decreased ability to experience pleasure; these are termed negative symptoms [5, 8]). While the occurrence of any of the aforementioned symptoms can help identify psychosis, their persistence over time instead indicates the onset of a psychotic disorder [5, 6]. One subtype of a psychotic disorder, ‘substance-induced psychotic disorder,’ is characterized by the emergence of psychotic symptoms triggered by psychoactive substances. Psychoactive substances are chemicals that alter brain activity and affect one’s mood, perception, or behavior [1]. Amphetamines are a type of psychoactive substance commonly prescribed for attention-deficit/hyperactivity disorder (ADHD), most often in the form of drugs like Adderall or Vyvanse [9].
Wired and Frayed: How Amphetamines Can Alter the Brain
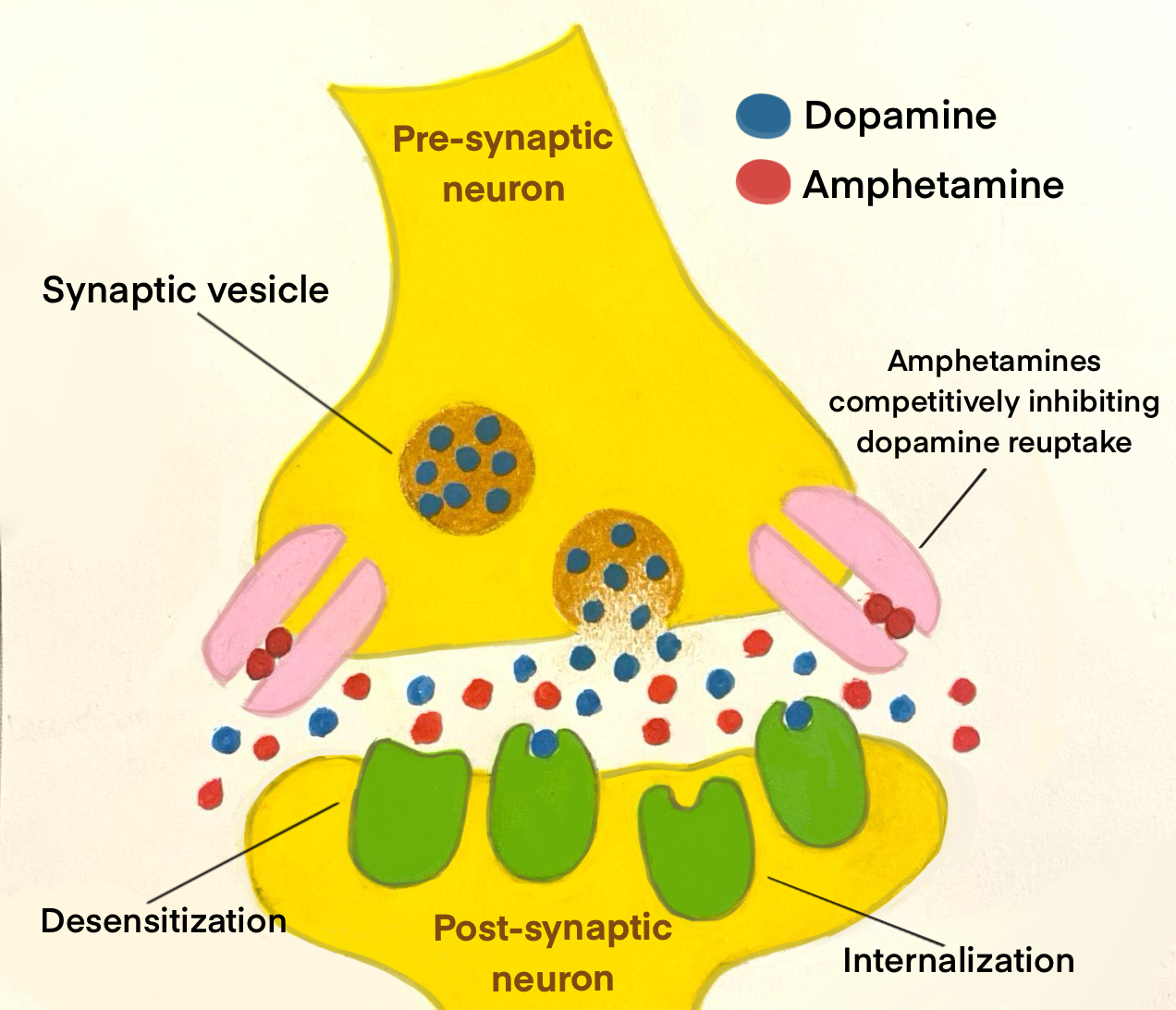
Amphetamine-induced psychotic disorder arises from the drug’s impact on chemical signaling molecules known as neurotransmitters [10]. In particular, amphetamines affect a class of neurotransmitters known as monoamines, which include serotonin, dopamine, norepinephrine, and epinephrine [10]. One important monoamine, dopamine, is known for its role in motivation and learning [11, 12]. Amphetamines increase dopamine release by nerve cells known as neurons and inhibit the reuptake of dopamine by these neurons [9]. Reuptake is the process by which neurotransmitters can be reabsorbed back into the neuron that released them [13]. Think of monoamine reuptake like a mail system. When a neuron sends out a message in the form of a neurotransmitter, that molecule will travel to a nearby receptor where it delivers its message. After the message is received, instead of letting the mail pile up, it gets ‘read’ and returned to the sender — the neuron — for future use. Reuptake ensures that the brain isn’t overwhelmed with too many messages at once. Reuptake helps regulate neurotransmitter levels and controls how long they can affect mood, attention, and other functions [14]. Therefore, when reuptake is inhibited, there is a surge in dopamine levels within key brain regions, including those responsible for reward processing and addiction [15]. Consistently high levels of dopamine from chronic amphetamine use can overstimulate networks of neurons in the brain that produce and transit dopamine, known as dopaminergic pathways [16]. Amphetamine specifically acts as a competitive inhibitor of dopamine reuptake, ‘competing’ with dopamine and blocking the dopaminergic neuron’s ability to reabsorb dopamine. This inhibition of reuptake over stimulates the dopamine system and contributes to the development of psychosis [16].
When dopamine levels remain elevated for prolonged periods, the brain attempts to counteract the overabundance of neurotransmitters through a process called downregulation [17]. In the case of amphetamine use, downregulation manifests primarily through desensitization, where dopamine receptors become less responsive to dopamine [18]. Another mechanism involved in downregulation is internalization, in which receptors are temporarily removed from the neuron’s surface to limit further dopamine stimulation [18]. Downregulation is an adaptive change that prevents receptors from being overwhelmed and reduces the brain’s responsiveness to dopamine, creating a cycle of tolerance where increasingly higher dopamine levels are needed to achieve the same effects [19]. Chronic amphetamine use, especially from a young age, can disrupt the creation of dopaminergic pathways to the prefrontal cortex — a region responsible for decision-making and emotional regulation [20, 21]. A disruption in dopaminergic pathways to the prefrontal cortex may cause less predictable reactions to situations and make navigating complex decisions or managing emotions far more challenging [20, 21]. The prefrontal cortex doesn't finish developing until our mid-20s, meaning disruptions to this region in adolescent brains can contribute to long-term impairments in cognitive control of memory and executive functions [20, 22].
In conjunction with dopamine, the brain's primary excitatory and inhibitory neurotransmitters — glutamate and GABA — play crucial roles in amphetamine-induced psychotic disorder [23]. Think of glutamate as the ‘gas pedal’ and GABA as the ‘brakes.’ Glutamate pushes things forward by exciting brain cells and increasing activity, while GABA pulls things back, slowing activity down [24]. Chronic amphetamine use floods the brain with dopamine, which can overstimulate the glutamate system, contributing to neural hyperactivity [23]. When amphetamines cause too much glutamate activity without enough GABA to keep it in check, it’s like driving a car with the brake lines cut: you’re more likely to crash, or, in this case, experience psychosis [25]. Specifically, elevated levels of dopamine increase the excitation of a type of glutamate receptors known as NMDA receptors [26, 27]. Concurrently, amphetamines also have an inhibitory effect on GABA neurons, amplifying the hyperactivity of dopamine neurons [7, 23]. The resulting glutamate-GABA imbalance forms a dangerous self-perpetuating feedback loop that also occurs in schizophrenia [20, 25].
Decoding Dopamine: The Neurochemistry of Schizophrenia
Schizophrenia is widely understood to have a neurochemical basis, with the dopamine hypothesis being one of the most classic models [28]. The dopamine hypothesis suggests that excessive dopaminergic activity, particularly in the mesolimbic pathway — a neural pathway that transports dopamine from the ventral tegmental area (VTA) to the nucleus accumbens, amygdala, and hippocampus — contributes to positive symptoms such as hallucinations and delusions [28, 29]. Think of dopamine as the volume control on a stereo: when turned up too high, the sound becomes distorted and the music starts to feel overwhelming. Heightened dopamine activity can overwhelm the brain’s communication systems, leading to a distorted perception of reality and, contributing to some of the symptoms of psychosis [28]. Conversely, in people with schizophrenia, the mesocortical dopamine pathway — which extends from the VTA to the prefrontal cortex — experiences a deficit of dopamine, which is associated with the majority of negative schizophrenic symptoms [29, 30].
Dopamine isn’t the only neurotransmitter that plays a role in the neurological basis of schizophrenia — reduced glutamate function has also been implicated in schizophrenia symptomatology [31]. Complementing the dopamine model, the glutamate hypothesis posits that dysfunction in NMDA receptors results in the underactivation of dopamine neurons in certain areas of the brain, leading to cognitive deficits and negative symptoms observed in people living with schizophrenia. [31]. Another important player in the neural basis of schizophrenia is serotonin, a neurotransmitter linked to mood regulation and cognition [32]. The serotonin hypothesis suggests that long-lasting depletion of serotonin may contribute to both positive and negative symptoms of schizophrenia [33]. Combined dysfunction of glutamate and serotonin impairs the regulation of dopaminergic neurons, contributing to disturbances in dopamine transmission observed in schizophrenia [31].
Blurred Lines: Navigating Overlap and Divergence
Amphetamine-induced psychotic disorder possesses numerous overlapping symptoms and neurochemical underpinnings with schizophrenia, making differential diagnosis challenging [34]. People living with both schizophrenia and amphetamine-induced psychotic disorder both exhibit positive symptoms such as hallucinations and delusions, typically linked to increases in dopamine release in their mesolimbic pathways [35]. One key difference between the two disorders is that stimulant-induced psychosis tends to present with a much higher proportion of positive symptoms than negative ones, whereas this disparity is not characteristic of schizophrenia [36, 37]. Individuals with either disorder may also experience disruptions in cognitive function, including impaired attention and decision-making abilities [25, 28]. In amphetamine-induced psychotic disorder, symptoms that emerge after chronic stimulant use typically subside after drug cessation. However, in schizophrenia, symptoms develop independently of drug use and follow a more chronic, lifelong trajectory for those affected [34, 38]. Although there are differences in etiology, both conditions involve the dysregulation of neurotransmitter signaling, particularly dopamine and glutamate [25, 28]. Positive symptoms of schizophrenia are thought to arise from abnormal dopamine activity, much like in amphetamine-induced psychotic disorder [25, 28]. Dysfunction of neurotransmitters can trigger psychotic episodes, which may persist even after drug discontinuation [49]. Interestingly, stimulant-induced psychotic disorder arose in less than 1% of adolescent and young adult patients receiving prescription stimulants, like amphetamines, for ADHD [9]. The dose and frequency of amphetamine use that can trigger a psychotic episode can vary for each individual [9]. After discontinuing use, symptoms of psychosis will typically cease after roughly thirty days [39]. However, about 20% of people who experience persistent psychotic symptoms are thought to be later diagnosed with schizophrenia [40]. People with schizophrenia tend to have higher rates of substance abuse compared to the general population [41]. For many individuals, substance abuse occurs before they develop psychotic symptoms, illustrating the complex relationship that exists between substance abuse and schizophrenia [41].
Primary psychotic disorders — or psychotic disorders that are not caused by substance use or other conditions — have many symptoms in common with substance-induced psychotic conditions, contributing to diagnostic challenges [1, 42]. Young adults between 19-30 have the highest rates of stimulant abuse and likelihood of developing substance-induced psychotic conditions, which coincides with the average onset age of schizophrenia: about 21–25 in males and 25–30 in females [42, 43]. Since both substance-induced psychotic conditions and primary psychotic disorders tend to arise in people’s 20s, diagnosing these conditions poses challenges [1, 42]. This challenge is exacerbated in college settings, which have the highest prevalence of prescription stimulant misuse and overall access to psychoactive substances [3, 4].
Never Tell Me the Odds: Risk Factors for Amphetamine-Induced Psychosis
College students are particularly vulnerable to prescription stimulant misuse and may feel pressured to enhance their academic performance or manage overwhelming workloads [44]. In fact, between 27-36% of students reported misusing their own prescription stimulants [3]. Even when prescribed, both long-term and high-dose amphetamine use can be very dangerous, as they elevate the likelihood of neurochemical disruptions that can trigger psychotic symptoms [25]. All in all, the annual prevalence of non-medical Adderall misuse among college students is higher than for age-matched individuals not enrolled in college [4]. Stimulant misuse, especially in environments like college campuses, can significantly increase the risk of experiencing a psychotic episode. College students can easily access stimulants, making misuse more likely to occur [4]. In addition, high-stress academic environments and poor sleep patterns can exacerbate the effects of stimulant misuse and further contribute to the onset of psychosis [25, 45].
Certain genetic and behavioral factors also increase susceptibility to developing amphetamine-induced psychotic disorder [2]. A family history of psychiatric disorders heightens both the probability of developing amphetamine-induced psychotic disorder and the duration of psychosis [2]. Individuals with a genetic predisposition to substance abuse may have a tendency to misuse any kind of substance, including prescription medications [46]. Therefore, individuals with a genetic predisposition to substance abuse may be at greater risk for developing substance-induced psychotic conditions [46]. Furthermore, stimulant misuse is often seen alongside other forms of substance abuse, such as marijuana or alcohol, which may also provoke or worsen psychotic symptoms [1, 47]. Those with a family history of psychotic disorders or substance abuse are at an increased risk of developing the disorder, with non-prescription amphetamine users being over five times more likely to experience psychosis compared to non-users [48]. Considering these risk factors, treatment and prevention of amphetamine-induced psychotic disorder must take into account each person’s individual susceptibility to developing the condition.
Mending the Mind: Approaches to Treatment & Prevention
Although stimulant use has been brought to light as a prominent issue, effective evidence-based treatment options remain limited [49]. Drug cessation and abstinence is considered the most effective strategy for both treatment and prevention of amphetamine-induced psychotic disorder, especially when combined with behavioral therapies [49]. However, achieving abstinence can be difficult, especially for individuals with co-occurring psychiatric disorders like ADHD, where stimulant misuse is linked to genetic predispositions for substance abuse [46]. Reducing drug use, rather than attempting complete abstinence, might be a more realistic goal for many; a reduction of drug use still improves mental health outcomes and reduces the risk of relapse for certain individuals with stimulant use disorders [50].
Traditionally, antipsychotic medications that modulate dopamine are used to treat amphetamine-induced psychotic disorder [38]. While they can offer short-term relief from hallucinations and delusions, antipsychotic medications are generally insufficient for long-term treatment use [2, 51]. Furthermore, antipsychotics carry a significant risk of adverse effects, including sedation, drowsiness, cognitive dulling, and exacerbation of depressive symptoms [52]. Antipsychotic drugs have been shown to increase the severity of depression and other mental health concerns, such as mood dysregulation and higher relapse rates [49]. As a result, antipsychotics may contribute to worsened outcomes for individuals with stimulant-use disorders. While antipsychotics may provide temporary stability, their risks must be weighed carefully on a case-to-case basis. Additionally, antipsychotics should only be used for short-term management and gradually tapered once symptoms subside [39, 49].
Behavioral interventions to help stop or reduce amphetamine misuse can either be implemented alongside antipsychotics to increase the efficacy of treatment, or provide a more sustainable alternative to pharmacological treatments [53]. If behavioral interventions are used alone, they can target the root of the stimulant-use disorder while avoiding the potential side effects of antipsychotics. Therapeutic strategies like cognitive behavioral therapy (CBT) and contingency management have been shown to help people manage psychosis more effectively in the long term and reduce stimulant misuse [53]. CBT helps people understand how their thoughts influence their feelings and actions to alter harmful thought patterns [54]. Contingency management rewards people with prizes or incentives for staying drug-free, reinforcing positive behavior and sobriety [55]. As interest in amphetamine-induced psychotic disorder is growing, more research is needed to establish the efficacy of different treatments for the disorder [53]. However, it is clear that achieving and maintaining abstinence from amphetamines is crucial to preventing future psychotic episodes [2]. Remaining abstinent is difficult due to the addictive nature of stimulants and is especially challenging in high-stress environments like college campuses, where academic pressures are immense and drugs are easily accessible [4, 45]. Strategies for reducing campus misuse, such as education programs and improved mental health resources, may mitigate risks [56]. While both pharmacological and behavioral treatments are useful in addressing amphetamine-induced psychotic disorder, a cautious approach to prescribing antipsychotics and their short-term usage is suggested [38]. Preferred long-term treatment solutions for amphetamine-induced psychotic disorder are behavioral therapies and harm reduction strategies, such as gradual reduction of stimulant use [49]. All in all, more research into amphetamine-induced psychotic disorder is necessary to develop effective and sustainable treatment methods.
Back to Reality: Insights and Implications
Amphetamine-induced psychotic disorder is a growing concern that causes serious psychological distress for those affected [1]. For individuals prescribed amphetamines, it is imperative for clinicians to stay up to date on research, keep their patients informed about risk factors and drug interactions, and monitor the mental well-being of their patients. The connection between amphetamine misuse on college campuses and the onset of psychotic disorders indicates a pressing need for awareness and targeted preventive measures [4, 45]. In light of the susceptibility of the college-aged population to both stimulant misuse and psychotic disorders, universities are uniquely positioned to address these overlapping issues [4, 45]. Future research can explore specialized interventions that incorporate both education and support systems. College-campus-based initiatives could more effectively and comprehensively address the risks of amphetamine misuse in this population, particularly given the intense academic pressures that often contribute to this behavior. Broadening our approach may inspire new ways to support student mental health and limit amphetamine-related harm across campuses.
Reference List
Fiorentini, A., Cantù, F., Crisanti, C., Cereda, G., Oldani, L., & Brambilla, P. (2021). Substance-induced psychoses: An updated literature review. Frontiers in Psychiatry, 12, 694863. doi:10.3389/fpsyt.2021.694863
Rognli, E.B., Bramness, J.G. (2015) Understanding the relationship between amphetamines and psychosis. Current Addiction Reports, 2, 285–292. doi:10.1007/s40429-015-0077-4
Edinoff, A. N., Nix, C. A., McNeil, S. E., Wagner, S. E., Johnson, C. A., Williams, B. C., Cornett, E. M., Murnane, K. S,; Kaye, A. M., & Kaye, A. D. (2022) Prescription stimulants in college and medical students: A narrative review of misuse, cognitive impact, and adverse effects. Psychiatry International, 3(3), 221-235. doi:10.3390/psychiatryint3030018
Welsh, J. W., Shentu, Y., & Sarvey, D. B. (2019). Substance use among college students. Focus,17(2), 117–127. doi:10.1176/appi.focus.20180037
Rosenthal, E.,& Ahmed, A.O. (2020). Psychosis. In: Zeigler-Hill, V., Shackelford, T.K. (Eds.) Encyclopedia of Personality and Individual Differences, 4196-4201. Springer, Cham. doi:10.1007/978-3-319-24612-3_934
Gaebel, W., & Zielasek, J. (2015). Focus on psychosis. Dialogues in Clinical Neuroscience, 17(1), 9–18. doi:10.31887/DCNS.2015.17.1/wgaebel
Heckers, S., & Konradi, C. (2015). GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia Research, 167(1-3), 4–11. https://doi.org/10.1016/j.schres.2014.09.041
American Psychiatric Association. (2022). Schizophrenia spectrum and other psychotic disorders. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). doi:10.1176/appi.books.9780890425787
Moran, L. V., Ongur, D., Hsu, J., Castro, V. M., Perlis, R. H., & Schneeweiss, S. (2019). Psychosis with methylphenidate or amphetamine in Patients with ADHD. The New England Journal of Medicine, 380(12), 1128–1138. doi:10.1056/NEJMoa1813751
Hasenhuetl, P. S., Bhat, S. ,Freissmuth, M., & Sandtner, W. (2019). A Concept for atypical monoamine transporter pharmacology. Molecular Pharmacology, 95(3) 303-312; doi:doi:10.1124/mol.118.114793
Mohebi, A, & Berke, J.D. (2020) Dopamine release drives motivation, independently from dopamine cell firing. Neuropsychopharmacology, 45(1). doi:10.1038/s41386-019-0492-7.
Juárez Olguín, H., Calderón Guzmán, D., Hernández García, E., & Barragán Mejía, G. (2016). The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxidative Medicine and Cellular Longevity, 2016(1). doi:10.1155/2016/9730467
Ilipilla, G., & Arnold, L. E. (2024). The role of adrenergic neurotransmitter reuptake inhibitors in the ADHD armamentarium. Expert Opinion on Pharmacotherapy, 25(8), 945–956. https://doi.org/10.1080/14656566.2024.2369197
Basmadjian, O. M., Occhieppo, V. B., Montemerlo, A. E., Rivas, G. A., Rubianes, M. D., Baiardi, G., & Bregonzio, C. (2024). Angiotensin II involvement in the development and persistence of amphetamine-induced sensitization: Striatal dopamine reuptake implications. European Journal of Neuroscience, 59(10), 2450–2464. doi:10.1111/ejn.16312
Harris, H. N., & Peng, Y. B. (2020). Evidence and explanation for the involvement of the nucleus accumbens in pain processing. Neural Regeneration Research, 15(4), 597–605. doi:10.4103/1673-5374.266909
Ashok, A. H., Mizuno, Y., Volkow, N. D., & Howes, O. D. (2017) Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: A systematic review and meta-analysis. JAMA Psychiatry, 74(5), 511–519. doi:10.1001/jamapsychiatry.2017.0135
Kohno, M., Dennis, L. E., McCready, H., & Hoffman, W. F. (2022). Dopamine dysfunction in stimulant use disorders: Mechanistic comparisons and implications for treatment. Molecular Psychiatry, 27(1), 220–229. doi:10.1038/s41380-021-01180-4
Nawaratne, V., McLaughlin, S. P., Mayer, F. P., Gichi, Z., Mastriano, A., & Carvelli, L. (2021). Prolonged amphetamine exposures increase the endogenous human dopamine receptors 2 at the cellular membrane in cells lacking the dopamine transporter. Frontiers in Cellular Neuroscience, 15. doi>10.3389/fncel.2021.681539
Koob, G. F., & Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. doi:10.1016/S2215-0366(16)00104-8
Sherrill, L. K., & Gulley, J. M. (2018). Effects of amphetamine exposure during adolescence on behavior and prelimbic cortex neuron activity in adulthood. Brain Research, 1694, 111–120. doi:10.1016/j.brainres.2018.05.028
Reynolds, L. M., Makowski, C. S., Yogendran, S. V., Kiessling, S., Cermakian, N., & Flores, C. (2015). Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a DCC-dependent manner. Neuropsychopharmacology, 40(5), 1101–1112. doi:10.1038/npp.2014.287
Orsini, C. A., Heshmati, S. C., Garman, T. S., Wall, S. C., Bizon, J. L., & Setlow, B. (2018). Contributions of medial prefrontal cortex to decision making involving risk of punishment. Neuropharmacology, 139, 205–216. doi:10.1016/j.neuropharm.2018.07.018
Jiao, D., Liu, Y., Li, X., Liu, J., & Zhao, M. (2015). The role of the GABA system in amphetamine-type stimulant use disorders. Frontiers in Cellular Neuroscience, 9, 162. doi:10.3389/fncel.2015.00162
Wu, C., & Sun, D. (2015). GABA receptors in brain development, function, and injury. Metabolic Brain Disease, 30(2), 367–379. doi:10.1007/s11011-014-9560-1
AlOtaibi, S., Emara, A., & Elsisi, H. (2024). Mechanisms of psychiatric disorders induced by amphetamines: A comprehensive review. International Journal of Science and Research Archive, 11(1), 260-274. doi:10.30574/ijsra.2024.11.1.0007.
Javitt D. C. (2023). Cognitive impairment associated with schizophrenia: From pathophysiology to treatment. Annual Review of Pharmacology and Toxicology, 63, 119–141. doi:10.1146/annurev-pharmtox-051921-093250
Li, M. H., Underhill, S. M., Reed, C., Phillips, T. J., Amara, S. G., & Ingram, S. L. (2017). Amphetamine and methamphetamine increase NMDAR-GluN2B synaptic currents in midbrain dopamine neurons. Neuropsychopharmacology, 42(7), 1539–1547. doi:10.1038/npp.2016.278
Brisch, R., Saniotis, A., Wolf, R., Bielau, H., Bernstein, H. G., Steiner, J., Bogerts, B., Braun, K., Jankowski, Z., Kumaratilake, J., Henneberg, M., & Gos, T. (2014). The role of dopamine in schizophrenia from a neurobiological and evolutionary perspective: Old fashioned, but still in vogue. Frontiers in Psychiatry, 5, 47. doi:10.3389/fpsyt.2014.00047
Doyle, C. A., & McDougle, C. J. (2021). Dopamine. In: Volkmar, F.R. (Eds.). Encyclopedia of Autism Spectrum Disorders. Springer, Cham. doi:10.1007/978-3-319-91280-6_823
McCutcheon, R. A., Abi-Dargham, A., & Howes, O. D. (2019). Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends in Neurosciences, 42(3), 205–220. doi:10.1016/j.tins.2018.12.004
Sotoyama, H. (2024). Putative neural mechanisms underlying release-mode-specific abnormalities in dopamine neural activity in a schizophrenia-like model: The distinct roles of glutamate and serotonin in the impaired regulation of dopamine neurons. European Journal of Neuroscience, 59(6), 1194–1212. doi:10.1111/ejn.16123
Lin, S. H., Lee, L. T., & Yang, Y. K. (2014). Serotonin and mental disorders: A concise review on molecular neuroimaging evidence. Clinical Psychopharmacology and Neuroscience, 12(3), 196–202. doi:10.9758/cpn.2014.12.3.196
Quednow, B. B., Geyer, M. A., Halberstadt, A. L. (2020). Chapter 39 - Serotonin and schizophrenia. In: Müller, C. P., & Cunningham, K. A. (Eds.). Handbook of Behavioral Neuroscience, 711-743. Elsevier. doi:10.1016/B978-0-444-64125-0.00039-6.
Ham, S., Kim, T. K., Chung, S., & Im, H. I. (2017). Drug abuse and psychosis: New insights into drug-induced psychosis. Experimental Neurobiology, 26(1), 11–24. doi:10.5607/en.2017.26.1.11
Howes, O., McCutcheon, R., & Stone, J. (2015). Glutamate and dopamine in schizophrenia: An update for the 21st century. Journal of Psychopharmacology (Oxford, England), 29(2), 97–115. doi:10.1177/0269881114563634
Correll, C. U., & Schooler, N. R. (2020). Negative symptoms in schizophrenia: A review and clinical guide for recognition, assessment, and treatment. Neuropsychiatric Disease and Treatment, 16, 519–534. doi:10.2147/NDT.S225643
Alexander, P. D., Gicas, K. M., Cheng, A. Lang, D. J., Procyshyn, R. M., Vertinsky, A. T., Panenka, W. J., Thornton, A. E., Rauscher, A., Wong, J. Y. X., Chan, T., Jones, A. A., Vila-Rodriguez, F., Honer, W. G., & Barr, A. M. (2019). A comparison of regional brain volumes and white matter connectivity in subjects with stimulant induced psychosis versus schizophrenia. Psychopharmacology, 236, 3385–3399. doi:10.1007/s00213-019-05298-w
Fluyau, D., Mitra, P., & Lorthe, K. (2019). Antipsychotics for amphetamine psychosis. A systematic review. Frontiers in Psychiatry, 10, 740. doi:10.3389/fpsyt.2019.00740
Baldaçara, L., Ramos, A., & Castaldelli-Maia, J. M. (2023). Managing drug-induced psychosis. International Review of Psychiatry, 35(5–6), 496–502. doi:10.1080/09540261.2023.2261544
Murrie, B., Lappin, J., Large, M., & Sara, G. (2020). Transition of substance-induced, brief, and atypical psychoses to schizophrenia: A systematic review and meta-analysis. Schizophrenia Bulletin, 46(3), 505–516. doi:10.1093/schbul/sbz102
Khokhar, J. Y., Dwiel, L. L., Henricks, A. M., Doucette, W. T., & Green, A. I. (2018). The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophrenia Research, 194, 78–85. doi:10.1016/j.schres.2017.04.016
Zhan, N., Sham, P. C., So, H. C., & Lui, S. S. Y. (2023). The genetic basis of onset age in schizophrenia: Evidence and models. Frontiers in Genetics, 14. doi:10.3389/fgene.2023.1163361
Board, A. R., Guy, G., Jones, C. M., & Hoots, B. (2020). Trends in stimulant dispensing by age, sex, state of residence, and prescriber specialty - United States, 2014–2019. Drug and Alcohol Dependence, 217(1). doi:10.1016/j.drugalcdep.2020.108297
Arria, A. M., Caldeira, K. M., Vincent, K. B., O'Grady, K. E., Cimini, M. D., Geisner, I. M., Fossos-Wong, N., Kilmer, J. R., & Larimer, M. E. (2017). Do college students improve their grades by using prescription stimulants nonmedically? Addictive Behaviors, 65, 245–249. doi:10.1016/j.addbeh.2016.07.016
Hajdúk, M., Dančík, D., Januška, J., Svetský, V., Straková, A., Turček, M., Vašečková, B., Forgáčová, Ľ., Heretik, A., & Pečeňák, J. (2020). Psychotic experiences in student population during the COVID-19 pandemic. Schizophrenia Research, 222, 520–521. doi:10.1016/j.schres.2020.05.023
Hatoum, A. S., Colbert, S. M. C., Johnson, E. C. Huggett, S. B., Deak, J. D., Pathak, G. A., Jennings, M. V., Paul, S. E., Karcher, N. R., Hansen, I., Baranger, D. A. A., Edwards, A., Grotzinger, A. D., Tucker-Drob, E. M., Kranzler, H. R., Davis, L. K., Sanchez-Roige, S., Polimanti, R., Gelernter, J., Edenberg, H. J., Bogdan, R., & Agrawal, A. (2023). Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nature Mental Health, 1, 210-223. doi:10.1038/s44220-023-00034-y
Cole, V. T., & Hussong, A. M. (2020). Psychosocial functioning among college students who misuse stimulants versus other drugs. Addictive Behaviors, 105. doi:10.1016/j.addbeh.2020.106290
Huang, C. L., Tsai, I. J., & Lee, C. W. (2022). Risk of psychosis in illicit amphetamine users: a 10 year retrospective cohort study. Evidence-based Mental Health, 25(4), 163–168. doi:10.1136/ebmental-2021-300300
Ronsley, C., Nolan, S., Knight, R., Hayashi, K., Klimas, J., Walley, A., Wood, E., & Fairbairn, N. (2020) Treatment of stimulant use disorder: A systematic review of reviews. PLoS ONE, 15(6). doi:10.1371/journal.pone.0234809
Amin-Esmaeili, M., Farokhnia, M., Susukida, R., Leggio, L., Johnson, R. M., Crum, R. M., & Mojtabai, R. (2024). Reduced drug use as an alternative valid outcome in individuals with stimulant use disorders: Findings from 13 multisite randomized clinical trials. Addiction, 119(5), 833–843. doi:10.1111/add.16409
Servonnet, A., & Samaha, A. N. (2020). Antipsychotic-evoked dopamine supersensitivity. Neuropharmacology, 163. doi:10.1016/j.neuropharm.2019.05.007
Groom, M. J., & Cortese, S. (2022). Current pharmacological treatments for ADHD. In: Stanford, S. C., & Sciberras, E. (Eds.). New Discoveries in the Behavioral Neuroscience of Attention-Deficit Hyperactivity Disorder. Springer, Cham. doi:10.1007/7854_2022_330
Harada, T., Tsutomi, H., Mori, R., & Wilson, D. B. (2018). Cognitive-behavioural treatment for amphetamine-type stimulants (ATS)-use disorders. The Cochrane Database of Systematic Reviews, 12(12). doi:10.1002/14651858.CD011315.pub2
Nakao, M., Shirotsuki, K., & Sugaya, N. (2021). Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. BioPsychoSocial Medicine, 15(1). doi:10.1186/s13030-021-00219-w
Rash, C. J., Stitzer, M., & Weinstock, J. (2017). Contingency management: New directions and remaining challenges for an evidence-based intervention. Journal of Substance Abuse Treatment, 72, 10–18. doi:10.1016/j.jsat.2016.09.008
Kennedy S. (2018). Raising awareness about prescription and stimulant abuse in college students through on-campus community involvement projects. Journal of Undergraduate Neuroscience Education, 17(1), A50–A53. PMID:30618499