Beauty is in the Brain of the Beholder: The Neuroscience Behind Aesthetic Perception
Alya Bagdas
Illustrations by Iona Duncan
The Pageant Winning Brain: Distinguishing Aesthetic Experiences
As you sit on your couch with the TV glaring in front of you, clips of a beauty pageant flash on the screen. You watch as contestants walk across the stage; they are dressed in outfits meticulously chosen to highlight their eyes or wearing makeup chosen to accentuate their sharp cheekbones. What makes the contestant with a symmetrical face and confident strut stand out? How will the contestants on TV influence the clothes you pick out to wear tomorrow or the way you consider your own attractiveness? While questions like these may seem obvious in the context of beauty pageants, they can be applied to many different daily experiences [1, 2]. Everything we see, taste, smell, hear, and touch manifests into experiences [1, 2, 3, 4]. As we process sensory information, we form subjective judgments, create emotional connections, and assign values that influence individual behavior [2, 5, 6]. For example, if you find people with full lips and brown hair more attractive, the contestants in the beauty pageant who have these features may appear more sympathetic and beautiful because of your preconceived notions of beauty [1, 2, 7].
Aesthetics as a philosophical field explores how each person cultivates their own aesthetic tastes, why tastes differ, and if one aesthetic taste is better or more ‘correct’ than another [8]. Neuroaesthetics, which is an interdisciplinary field, applies these complex questions to the brain, investigating the neurological and behavioral processes that impact aesthetic taste and experience. Though these processes are not fully understood, previous life experiences, emotions, and perceptions all guide and influence our interpretation of the present [2, 9]. Neuroaesthetics allows us to examine the neurological underpinnings of aesthetic experiences and preferences from the individual level to our broader society [2].
The Science Behind the Spark
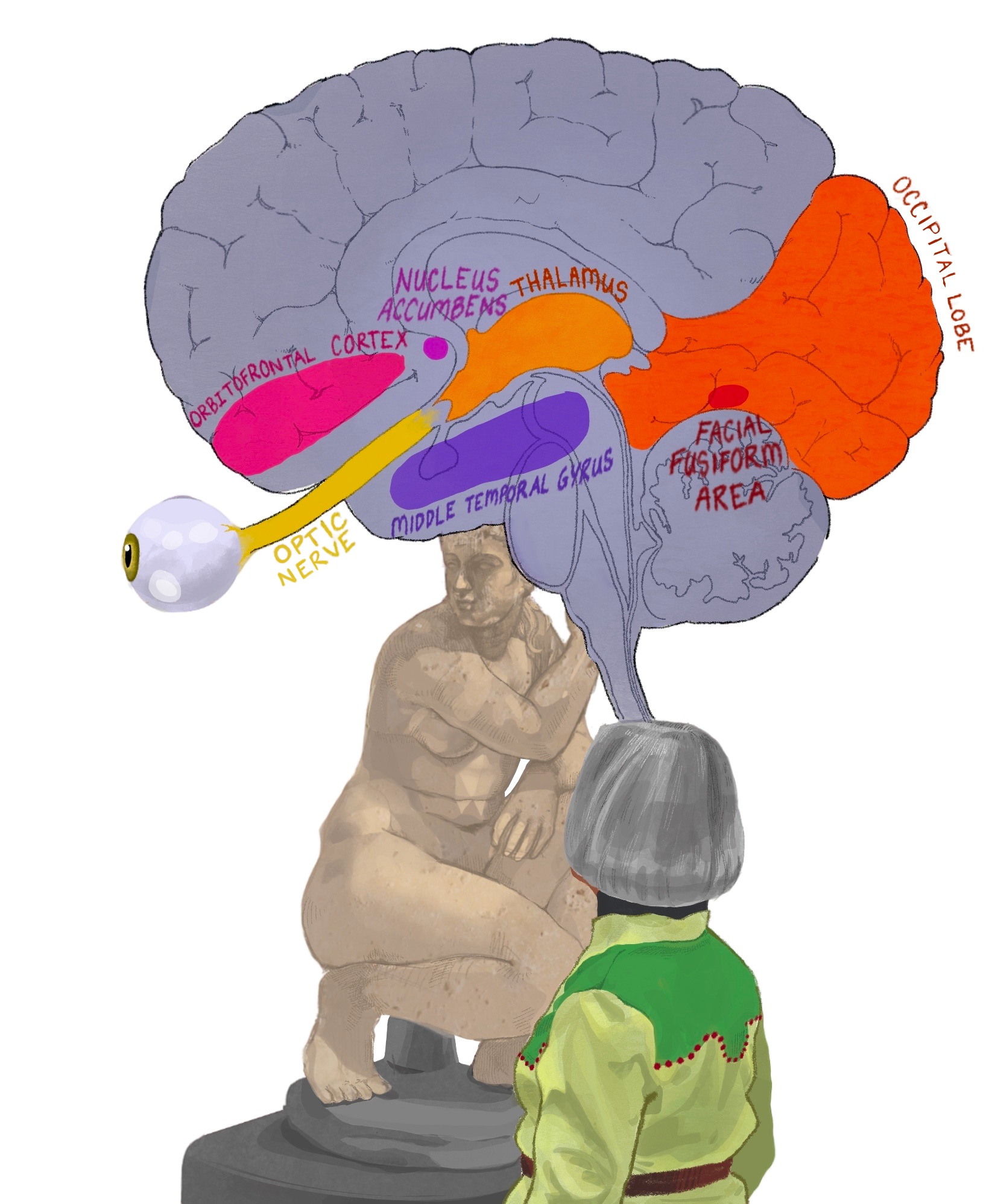
When you meet someone for the first time, you immediately begin to form an impression of them based on your aesthetic evaluation [10]. The symmetry of their face, the brightness of their eyes, the contour of their jawline, and even the shifting of their facial expressions can contribute to your subconscious assessment of their appearance. In fact, it only takes about 100 milliseconds of exposure to a face to judge whether or not it is attractive. However, it is important to note that this first impression is malleable [10]. The visual processing of a face begins when light enters the eye and hits the retina, which transmits light into an electrical signal that is understood by the brain [2, 11, 12]. Electrical signals are the means through which neurons, the fundamental cells of the nervous system, communicate with one another [13]. In the case of our visual system, neurons in the optic nerve carry electrical signals from the retina to the brain [14]. From the optic nerve, electrical signals pass through the thalamus — the relay center, similar to a train station — to be directed to different regions of the brain. For visual processing, information is sent to the occipital lobe [11, 12]. The occipital lobe forms a basic image of the person’s face, accounting for details like the edges of their jaw, the orientation of their head, the placement of their eyebrows, and the color of their hair [11, 12]. Electrical signals then travel to different parts of the brain via networks of neurons to complete higher-order processes, which are cognitive functions that integrate sensory information into thoughts and actions [11, 12, 15].
To carry out higher-order processing, visual information from the occipital lobe needs to be delivered to crucial brain regions [16, 17]. One of these regions is the fusiform face area (FFA), which allows us to recognize and distinguish different faces by processing the location of facial features and their variation across people [16, 17]. When we encounter an unfamiliar face for the first time, we unconsciously perform distinct eye movements that scan the face to gather visual information [15, 18]. We distinguish individual differences between people’s faces in the FFA, such as the distance between their eyes, the angle of their brows, and the spacing of their cheeks [15, 17]. The orbitofrontal cortex (OFC) integrates sensory information from the occipital lobe, the FFA, and the limbic system — a group of brain structures that regulate emotions, memory, and behavior [15, 17, 19, 20]. Integration of sensory information allows the OFC to associate certain visual aspects with rewarding properties [15, 17, 19, 20]. The OFC plays a critical role in assigning value to aesthetic stimuli, labeling any received input as positive, neutral, or negative [2, 21, 22]. Neurons from the OFC transmit signals to the nucleus accumbens (NAc), causing altering the release of a chemical messenger called dopamine, which is critical in regulating our reward system [2, 15, 20, 23, 24]. When we look at attractive faces or other rewarding stimuli, the OFC sends excitatory signals to the NAc, which leads to increased dopamine release in the NAc [15, 25].
The dopamine reward system is activated in response to seeing beautiful stimuli, contributing to a feeling of reward and reinforcing that certain objects and faces are beautiful [26, 27, 28]. When a certain cue results in an increase of dopamine release in the NAc, that cue becomes associated with a reward and that person is more motivated to seek out that particular cue in the future or carry out a certain behavior that will allow them to experience another rewarding feeling [29, 30, 31]. For example, if we have more positive interactions with people who look a certain way, dopamine reinforces that it was a rewarding experience, encouraging us to continue to seek out other individuals who look similar to them [31, 32, 33, 34]. Additionally, dopamine release in the NAc can impact the prominence of the cue being processed [26]. An increase in dopamine can essentially ‘tag’ certain faces or aesthetic experiences as more significant, and therefore they are more likely to be remembered [29, 30, 31].
Another part of aesthetic evaluation unfolds in the middle temporal gyrus (MTG) [2, 30, 35]. Although the MTG is not traditionally considered one of the core areas of facial processing, it plays a crucial role in integrating inputs from our visual system, memory, emotions, and social context [2, 30, 35, 36]. When we judge someone's attractiveness, the MTG helps us synthesize not only what we see but also how we feel about them based on prior experiences and learned information [2, 37, 38]. In essence, the MTG allows us to interpret faces beyond simple visual features by incorporating emotional significance and social meaning into our judgment of attractiveness [2]. For example, when you’re looking at someone, the MTG is activated during your evaluation of their attractiveness, and the MTG may be drawing from memories of previous encounters with individuals who share similar traits. Perhaps those past experiences were positive, causing the brain’s reward systems to link those features with a rewarding feeling. Therefore, the MTG may help encode learned social and cultural values about beauty, combining objective visual stimuli with subjective emotional and social context [2].
Beauty and the Behavior
Various theories link the reward system's role in aesthetic experiences to specific behaviors. One popular theory focuses on the evolutionary and reproductive benefits of finding certain features more rewarding than others [39, 40, 41]. Perceived physical and facial attractiveness can symbolize fertility, health, and genetic favorability, impacting the initiation of sexual relations and the continued motivation for parental behavior [39, 40, 41]. For example, facial masculinity in men, like sharp jaws and thick eyebrows, is perceived as a sign of good health and genes [42]. Perceived notions of favorability, however, do not always have a direct link to health; a common example is that facial structures, like symmetry, have no identifiable correlations to one’s health status, but we still utilize these structures to judge other’s health status and attractiveness [42, 43]. People of different sexualities demonstrated greater activation of their OFC when shown faces of individuals of the sex they were attracted to compared to faces of individuals of the sex they were not attracted to [39, 41, 44]. Heterosexual people demonstrated greater activation of components of the brain’s reward systems — namely the NAc, OFC, and the prefrontal cortex — when shown faces of members of the opposite sex that they categorized as attractive [39]. This indicates that the neurophysiology underlying our romantic choices and the reward value of attractiveness is similar across sexes [39, 41]. In addition to mate-choice behaviors, attractiveness also influences caregiver behavior toward infants [39, 41]. ‘Cuter’ infants are more likely to receive care and positive attention from others [41]. Perception of cuteness leading to increased attention might be the result of cuteness being associated with health and viability, where the caregiver's attention to cuter babies is more likely to ‘pay off’ in the survival of the child [39]. For example, when a woman looks at a baby they consider ‘cute’, their NAc, and reward system, becomes activated, resulting in the reinforcement of those ‘cute’ features as favorable [39]. Differences in aesthetic processing between sexes are of great interest in the field of neuroaesthetics, with ongoing efforts to uncover the neural pathways, differences, and behaviors underlying mate choice and infant care choices [15, 45, 46, 47].
Another large focus within the field of neuroaesthetics is the cycle of influence between individual aesthetic ideals and larger societal ideals [48]. Societal standards of beauty directly influence how our brains process and evaluate attractiveness [48, 49]. Western beauty standards often emphasize extreme thinness for women and hypermuscularity for men while emphasizing Anglo-European features, creating a narrow definition of what is deemed attractive [48, 49]. Society’s fixation on specific body types is evident within the media, which frequently presents disproportionate representations of appearance and physique. Different forms of entertainment and social media bombard people with idealized images of physical appearance [48, 49, 50, 51, 52]. Photoshopped images present even more unrealistic representations of beauty, creating an environment where individuals increasingly endorse socially prescribed appearance ideals. Repeated exposure to these ideals can condition the brain to associate those traits with higher social value and reward [30, 31, 48, 49]. Over time, the brain may develop a preference for traits that are constantly being portrayed as desirable, driven by the release of dopamine and memory processing [2, 30, 31]. The internalization of societal beauty ideals leads people to engage in behaviors aimed at conforming to these standards, such as dieting, excessive exercise, and cosmetic surgery [49, 50, 51, 52]. Dissatisfaction with one’s own attractiveness is particularly prevalent among women, who are subject to stricter and more rigid social standards than men [53]. Deviations from societal beauty standards can trigger feelings of social rejection or inadequacy, which may activate brain regions associated with negative emotional states, such as the anterior insula or amygdala, intensifying the pressure we feel to conform [54, 55].
Cultural and social backgrounds can also impact our perception of what is beautiful [49]. While many socioeconomic groups may share similar standards of facial attractiveness — such as symmetry and youth — notable differences in what others deem attractive persist and are shaped by their race, ethnicity, and gender [42, 43, 49]. For instance, Black women tend to be more accepting of body diversity, despite pressures they may face linked to colorism — bias favoring lighter skin tones — in determining beauty [49]. Factors such as colorism and hair texture play crucial roles in shaping perceptions of beauty within different communities; women’s attractiveness ratings are often influenced by these characteristics [49, 53]. Furthermore, societal implications of beauty extend beyond personal perception; they influence social interactions, professional opportunities, and social standing [49, 56]. The ‘beautiful-is-good’ stereotype suggests that attractive individuals are often perceived as more intelligent, competent, and cooperative, leading to biases in various settings, including hiring practices and legal judgments [49, 56]. For example, women are likely to offer more resources to attractive males in behavioral games, demonstrating that physical appearance can affect social dynamics and decision-making [56]. The ‘beautiful-is-good’ attractiveness bias reinforces the notion that those who align more closely with conventional beauty standards tend to receive advantages in employment opportunities and income, perpetuating a cycle in which beauty equates to social and economic capital [49, 56].
A Painting is Worth a Thousand Neurons
Watching a beauty pageant may seem like a simple action on the surface, but on a deeper level, it involves the merging of all the aesthetic values that surround you and the intricate interactions of neurons within your brain [2, 48]. Neuroaesthetics uncovers the hidden choreography of the social, individual, and neural relationships that are behind the judgments and actions that inform our daily experiences. Our personal notions of beauty are behind our daily choices and perceptions. Every brushstroke in a painting, every note in a song, every face in a crowd becomes part of the mental landscape where aesthetics and our brains intertwine. By understanding the neuroscience behind our aesthetic experiences, we gain insight into the art of our own perceptions, an art we are painting in every choice we make, every moment we savor, and every beautiful thing we see [2, 3, 4, 9, 48].
Reference List
Thiruchselvam, R., Harper, J., & Homer, A. L. (2016). Beauty is in the belief of the beholder: Cognitive influences on the neural response to facial attractiveness. Social cognitive and Affective Neuroscience, 11(12), 1999-2008. doi:10.1093/scan/nsw115
Chatterjee, A., & Vartanian, O. (2014). Neuroaesthetics. Trends in Cognitive Sciences, 18(7), 370-375. doi:10.1016/J.TICS.2014.03.003
Becker, S., Bräscher, A.-K., Bannister, S., Bensafi, M., Calma-Birling, D., Chan, R. C. K., Eerola, T., Ellingsen, D.-M., Ferdenzi, C., Hanson, J. L., Joffily, M., Lidhar, N. K., Lowe, L. J., Martin, L. J., Musser, E. D., Noll-Hussong, M., Olino, T. M., Pintos Lobo, R., & Wang, Y. (2019). The role of hedonics in the human affectome. Neuroscience & Biobehavioral Reviews, 102, 221-241. doi:10.1016/j.neubiorev.2019.05.003
Jacobi, C., Varga, P. J., Jessani, Z., & Vaidyanathan, B. (2024). Individual differences in scientists' aesthetic disposition, aesthetic experiences, and aesthetic sensitivity in scientific work. Frontiers in psychology, 14. doi:10.3389/fpsyg.2023.1197870
Han, S., Liu, S., Gan, Y., Xu, Q., Xu, P., Luo, Y., & Zhang, L. (2020). Repeated exposure makes attractive faces more attractive: Neural responses in facial attractiveness judgment. Neuropsychologia, 139. doi:10.1016/j.neuropsychologia.2020.107365
Kenett, Y. N., Cardillo, E. R., Christensen, A. P., & Chatterjee, A. (2023). Aesthetic emotions are affected by context: A psychometric network analysis. Scientific Reports, 13(1), 1-16. doi:10.1038/s41598-023-48219-w
Talamas, S. N., Mavor, K. I., & Perrett, D. I. (2016). Blinded by beauty: Attractiveness bias and accurate perceptions of academic performance. PLoS ONE, 11(2). doi:10.1371/JOURNAL.PONE.0148284
Page, J. (2022). Aesthetic understanding. Estetika: The European Journal of Aesthetics, 51(9), 48-68. doi:10.33134/eeja.269
Karim, A. K. M. R., Proulx, M. J., de Sousa, A. A., & Likova, L. T. (2022). Do we enjoy what we sense and perceive? A dissociation between aesthetic appreciation and basic perception of environmental objects or events. Cognitive, Affective & Behavioral Neuroscience, 22(5), 904-951. doi:10.3758/s13415-022-01004-0
Hung, S. M., Nieh, C. H., & Hsieh, P. J. (2016). Unconscious processing of facial attractiveness: Invisible attractive faces orient visual attention. Scientific Reports, 6(1), 1-8. doi:10.1038/srep37117
Wang, L., Ma, L., Yang, J., & Wu, J. (2021). Human somatosensory processing and artificial somatosensation. Cyborg and Bionic Systems, 2021. doi:10.34133/2021/9843259
Wu, Y. H., Podvalny, E., Levinson, M., & He, B. J. (2024). Network mechanisms of ongoing brain activity’s influence on conscious visual perception. Nature Communications, 15(1), 1-18. doi:10.1038/s41467-024-50102-9
Catterall, W. A., Wisedchaisri, G., & Zheng, N. (2017). The chemical basis for electrical signaling. Nature Chemical Biology, 13(5), 455-463. doi:10.1038/nchembio.2353
Cowan, C. S., Renner, M., De Gennaro, M., Gross-Scherf, B., Goldblum, D., Hou, Y., Munz, M., Rodrigues, T. M., Krol, J., Szikra, T., Cuttat, R., Waldt, A., Papasaikas, P., Diggelmann, R., Patino-Alvarez, C. P., Galliker, P., Spirig, S. E., Pavlinic, D., Gerber-Hollbach, N., Schuierer, S., Srdanovic, A., Balogh, M., Panero, R., Kusznerik, A., Syabo, A., Stadler, M. B., Orgül, S., Picelli, S., Hasler, P. W., Hierlemann, A., Scholl, H. P. N., Roma, G., Nigsch, F. & Roska, B. (2020). Cell types of the human retina and its organoids at single-cell resolution. Cell, 182(6), 1623–1640.e34. doi:10.1016/j.cell.2020.08.013
Schendan, H.E. (2019). Memory influences visual cognition across multiple functional states of interactive cortical dynamics. Psychology of Learning and Motivation, 71, 303-386. doi:10.1016/bs.plm.2019.07.007
Yarosh, D. B. (2019). Perception and deception: Human beauty and the brain. Behavioral Sciences, 9(4), 34. doi:10.3390/BS9040034
Kho, S. K., Wong, H. K., & Estudillo, A. J. (2023). Investigating the role of the fusiform face area and occipital face area using multifocal transcranial direct current stimulation. Neuropsychologia, 189(1). doi:10.1016/j.neuropsychologia.2023.108663
Kanan, C., Bseiso, D. N. F., Ray, N. A., Hsiao, J. H., & Cottrell, G. W. (2015). Humans have idiosyncratic and task-specific scanpaths for judging faces. Vision Research, 108, 67-76. doi:10.1016/j.visres.2015.01.013
Rudebeck, P.H., & Rich, E.L. (2018). Orbitofrontal cortex. Current Biology, 28(18), R1083-R1088. doi:10.1016/j.cub.2018.07.018
Rolls, E. T. (2023). Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Structure and Function, 228(5), 1201-1257. doi:10.1007/S00429-023-02644-9
Eng, G. K., Sim, K., & Chen, S. H. A. (2015). Meta-analytic investigations of structural grey matter, executive domain-related functional activations, and white matter diffusivity in obsessive compulsive disorder: An integrative review. Neuroscience & Biobehavioral Reviews, 52(1), 233–257. doi:10.1016/j.neubiorev.2015.03.002
Martín-Loeches, M., Hernández-Tamames, J. A., Martín, A., & Urrutia, M. (2014). Beauty and ugliness in the bodies and faces of others: An fMRI study of person esthetic judgement. Neuroscience, 277(1), 486–497. doi:10.1016/j.neuroscience.2014.07.040
Juárez Olguín, H., Calderón Guzmán, D., Hernández García, E., & Barragán Mejía, G. (2016). The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxidative Medicine and Cellular Longevity, 2016(1). doi:10.1155/2016/9730467
Zhao, X., Wang, J., Li, J., Luo, G., Li, T., Chatterjee, A., Zhang, W., & He, X. (2020). The neural mechanism of aesthetic judgments of dynamic landscapes: An fmri study. Scientific Reports, 10(1), 20774. doi:10.1038/s41598-020-77658-y
Ueda, R., & Abe, N. (2021). Neural representations of the committed romantic partner in the nucleus accumbens. Psychological Science, 32(12), 1884–1895. doi:10.1177/09567976211021854
Kutlu, M. G., Zachry, J. E., Melugin, P. R., Cajigas, S. A., Chevee, M. F., Kelly, S. J., Kutlu, B., Tian, L., Siciliano, C. A., & Calipari, E. S. (2021). Dopamine release in the nucleus accumbens core signals perceived saliency. Current biology, 31(21), 4748–4761. doi:10.1016/j.cub.2021.08.052
Hu, C. P., Huang, Y., Eickhoff, S. B., Peng, K., & Sui, J. (2020). Seeking the “beauty center” in the brain: A meta-analysis of fmri studies of beautiful human faces and visual art. Cognitive, Affective, & Behavioral Neuroscience, 20(6), 1200–1215. doi:10.3758/s13415-020-00827-z
Salomon, T., Botvinik-Nezer, R., Oren, S., & Schonberg, T. (2020). Enhanced striatal and prefrontal activity is associated with individual differences in nonreinforced preference change for faces. Human Brain Mapping, 41(4), 1043–1060. doi:10.1002/hbm.24859
Chen, J., & Bruchas, M. (2021). Neuromodulation: A model for dopamine in salience encoding. Current Biology, 31(21), R1426–R1429. doi:0.1016/j.cub.2021.09.038
Ota, C., & Nakano, T. (2021). Self-face activates the dopamine reward pathway without awareness. Cerebral Cortex, 31(10), 4420–4426. doi:10.1093/cercor/bhab096
Lewis, A. F., Bohnenkamp, R., Myers, M., den Ouden, D. B., Fritz, S. L., & Stewart, J. C. (2024). Effect of positive social comparative feedback on the resting state connectivity of dopaminergic neural pathways: A preliminary investigation. Neurobiology of Learning and Memory, 212(1). doi:10.1016/j.nlm.2024.107930
Schultz W. (2015). Neuronal reward and decision signals: from theories to data. Physiological reviews, 95(3), 853–951. doi: 10.1152/physrev.00023.2014
Gluth, S., Hotaling, J. M., & Rieskamp, J. (2016). The attraction effect modulates reward prediction errors and intertemporal choices. The Journal of Neuroscience, 37(2), 371–382. doi: 10.1523/jneurosci.2532-16.2016
Songur, A. (2023). Neuroanatomy of romantic love. Scripta Medica, 54(3), 289–295. doi: 10.5937/scriptamed54-45541
Smith, D. V., Clithero, J. A., Boltuck, S. E., & Huettel, S. A. (2014). Functional connectivity with ventromedial prefrontal cortex reflects subjective value for social rewards. Social Cognitive and Affective Neuroscience, 9(12), 2017–2025. doi:10.1093/scan/nsu005
Davey, J., Thompson, H. E., Hallam, G., Karapanagiotidis, T., Murphy, C., De Caso, I., Krieger-Redwood, K., Bernhardt, B. C., Smallwood, J., & Jefferies, E. (2016). Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. NeuroImage, 137(1), 165–177. doi:10.1016/j.neuroimage.2016.05.051
Isik, A. I., & Vessel, E. A. (2021). From visual perception to aesthetic appeal: Brain responses to aesthetically appealing natural landscape movies. Frontiers in Human Neuroscience, 15. doi:10.3389/FNHUM.2021.676032/BIBTEX
Rasche, S. E., Beyh, A., Paolini, M., & Zeki, S. (2024). Neural correlates of the experience of ugliness. European Journal of Neuroscience, 60(7), 5671-5679. doi:10.1111/ejn.16517
Comfort, W. E., & Freitas, A. L. (2022). The neuroscience of beauty. In: Bogio, P. S., Wingenbach, T. S. H., da Silveira Coêlho, M. L., Comfort, W. E., Marques, L. M., & Alves, M. V. C. (Eds.) Social and Affective Neuroscience of Everyday Human Interaction, 53–60. Springer Nature. doi:10.1007/978-3-031-08651-9_4
Chatterjee, A., & Vartanian, O. (2016). Neuroscience of aesthetics. Annals of the New York Academy of Sciences, 1369(1), 172–194. doi:10.1111/nyas.13035
Hahn, A. C., & Perrett, D. I. (2014). Neural and behavioral responses to attractiveness in adult and infant faces. Neuroscience and biobehavioral reviews, 46(4), 591–603. doi:10.1016/j.neubiorev.2014.08.015
Henderson, A. J., Holzleitner, I. J., Talamas, S. N., & Perrett, D. I. (2016). Perception of health from facial cues. Philosophical Transactions of the Royal Society B: Biological Sciences, 371. doi:10.1098/rstb.2015.0380
Foo, Y. Z., Simmons, L. W., & Rhodes, G. (2017). Predictors of facial attractiveness and health in humans. Scientific Reports, 7(1). doi:10.1038/srep39731
Tieo, S., Dezeure, J., Cryer, A., Pascal Lepou, Marie J.E. Charpentier, & Renoult, J. P. (2023). Social and sexual consequences of facial femininity in a non-human primate. IScience, 26(10), 107901–107901. doi:10.1016/j.isci.2023.107901
Buss, D. M., & Schmitt, D. P. (2019). Mate Preferences and Their Behavioral Manifestations. Annual Review of Psychology, 70(1), 77–110. doi:10.1146/annurev-psych-010418-103408
Cazzato, V., Mele, S., & Urgesi, C. (2014). Gender differences in the neural underpinning of perceiving and appreciating the beauty of the body. Behavioural Brain Research, 264(1), 188–196. doi:10.1016/j.bbr.2014.02.001
Paul, S., Austin, J., Elliott, R., Ellison-Wright, I., Wan, M. W., Drake, R., Downey, D., Elmadih, A., Mukherjee, I., Heaney, L., Williams, S., & Abel, K. M. (2018). Neural pathways of maternal responding: Systematic review and meta-analysis. Archives of Women's Mental Health, 22(2), 179–187. doi:10.1007/s00737-018-0878-2
Pearce, M. T., Zaidel, D. W., Vartanian, O., Skov, M., Leder, H., Chatterjee, A., & Nadal, M. (2016). Neuroaesthetics: The cognitive neuroscience of aesthetic experience. Perspectives on Psychological Science, 11(2), 265-279. doi:10.1177/1745691615621274
Rodgers, R. F., Campagna, J., & Attawala, R. (2019). Stereotypes of physical attractiveness and social influences: The heritage and vision of Dr. Thomas Cash. Body Image, 31, 273-279. doi:10.1016/J.BODYIM.2019.01.010
Aparicio-Martinez, P., Perea-Moreno, A. J., Martinez-Jimenez, M. P., Redel-Macías, M. D., Pagliari, C., & Vaquero-Abellan, M. (2019). Social media, thin-ideal, body dissatisfaction and disordered eating attitudes: An exploratory analysis. International Journal of Environmental Research and Public Health, 16(21). doi:10.3390/IJERPH16214177
Pedalino, F., & Camerini, A. L. (2022). Instagram use and body dissatisfaction: The mediating role of upward social comparison with peers and influencers among young females. International Journal of Environmental Research and Public Health, 19(3). doi:10.3390/IJERPH19031543
Kaziga, R., Muchunguzi, C., Achen, D., & Kools, S. (2021). Beauty is skin deep; The self perception of adolescents and young women in construction of body image within the Ankole Society. International Journal of Environmental Research and Public Health, 18(15). doi:10.3390/IJERPH18157840
Swami, V., Weis, L., Barron, D., & Furnham, A. (2018). Positive body image is positively associated with hedonic (emotional) and eudaimonic (psychological and social) well-being in British adults. The Journal of Social Psychology, 158(5), 541–552. doi:10.1080/00224545.2017.1392278
Porcelli, S., Van Der Wee, N., van der Werff, S., Aghajani, M., Glennon, J. C., van Heukelum, S., Mogavero, F., Lobo, A., Olivera, F. J., Lobo, E., Posadas, M., Dukart, J., Kozak, R., Arce, E., Ikram, A., Vorstman, J., Bilderbeck, A., Saris, I., Kas, M. J., & Serretti, A. (2019). Social brain, social dysfunction and social withdrawal. Neuroscience & Biobehavioral Reviews, 97(97), 10–33. doi:10.1016/j.neubiorev.2018.09.0121
MacCallum, F., & Widdows, H. (2018). Altered images: Understanding the influence of unrealistic images and beauty aspirations. Health Care Analysis, 26(3), 235-245. doi:10.1007/S10728-016-0327-1/METRICS
Shang, J., & Zhang, Y. (2024). Influence of male’s facial attractiveness, vocal attractiveness and social interest on female’s decisions of fairness. Scientific Reports, 14(1), 1-11. doi:10.1038/s41598-024-67841-w